How To Draw Atomic And Molcular Orbitals
Introduction to Molecular Orbital Theory
This collection of spider web documents can be used every bit a "backup" to Henry Rzepa'south on-line Pericyclic Chemical science form. Information technology uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction mechanisms - such as those found in pericyclic chemistry. The pictorial content uses both Chimed and VRML enhanced images, and demonstrates that these forms can be used in the place of traditional "curly arrows" and "resonance hybrids", as they can provide a deeped and more subtle insight into the mechanism of a reaction.
Index
- Introduction
- Atomic and Molecular Orbitals
- Orbitals for selected molecules
i. Introduction
Modern chemistry has depended upon the apply of models of increasing comlexity. Atoms can exist represented as spheres connected past cyclinders or sticks. In gild to empathise the mechanism of many reactions, Lewis Theory, adult by Robinson and Ingold, can provide a succesful answer.Lewis Theory uses curly arrows to announce electron migration during a chemic reaction and has led to a greater understanding of the factors controlling chemic reactions.
Pauling with others, adult Resonance Theory, which provided the rationale to an all-embracing orbital theory. The use of "canonical forms" and "resonance hybrids", alonng with extensive use of curvy arrows has provided the fundamental background to modern organic theory, just for eg. Diels-Alder and pericyclic reactions, the curly arrow format is not very clear and in some instances the reactions are described every bit no-machanism reactions. Woodward and Hoffmann showed that by examining the interaction of the frontier molecular orbitals (ie. the Highest Occupied, Human and Everyman Unoccupied, LUMO) both the regio- and stereospecificity could exist accountred for.
Woodward and Hoffmann work was assimilated into general organic reaction theory.
2. Atomic and Molecular Orbitals
By sharing electron, molecules can form bonds, and it is possible to regard the sharing of 2 electrons by two atoms every bit constituting a chemical bond. Atoms tin can share one, two or iii electrons (forming unmarried, double and triple bonds).A hydrogen atom consists of a nucleus (a proton) and an electron. It is not possible to accurately decide the position of the electron, just it is possible to calculate the probability of findng the electron at any point around the nucleus. With a hydrogen atom the probability distribution is spherical effectually the nucleus and it is possible to draw a spherical boundary surface, inside which there is a 95% possibility of finding the electron. The electron has a fixed energy and a fixed spatial distribution chosen an orbital. In the helium atom at that place are two electrons associated with the helium nucleus. The electrons have the aforementioned spatial distribution and free energy (ie. they occupy the same orbital), but they differ in their spin (Pauli exlusion principle). In general: electrons in atomic nuclei occupy orbitals of stock-still energy and spatial distribution, and each orbital only contains a maximum of two electrons with anti-parallel spins.
In physics, periodic phenomena are associated with a "wave equation", and in atomic theory the relevant equation is chosen the "Schrödinger Equation". The wave equation predicts discrete solutions in one dimension for a particle confined to a box with infinite walls, The solutions can be shown as in the effigy beneath:
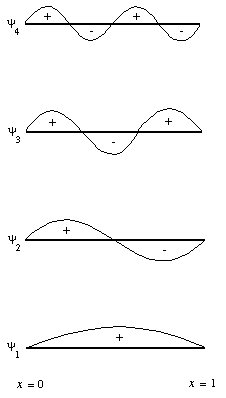
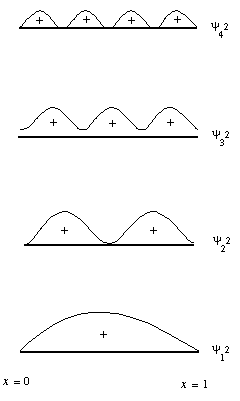
y 1 - y 4 represent solutions of increasing energy. In iii dimensions, the equation determines the energy and defines the spatial distribution of each electron. Solutions of the wave equations in three-dimensions allows calculation of the "shape" of each orbital. The first five solutions of the moving ridge equation for an electron associated with a proton can be shown in the effigy below:
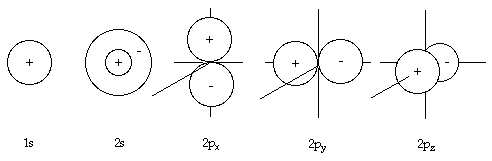
In the hydrogen atom, the 1s atomic orbital has the everyman energy, while the remainder (2s, 2px, 2py and 2pz ) are of equal energy (ie.degenerate), merely for all other atoms, the 2s diminutive orbital is of lower enegry than the 2p10, 2py and 2pz orbitals, which are degenerate.
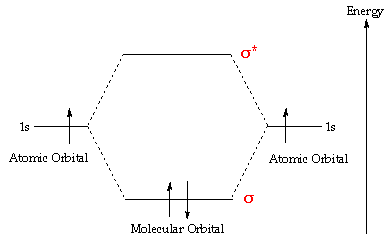
In atoms, electrons occupy atomic orbitals, only in molecules they occupy similar molecular orbitals which surround the molecule. The simplest molecule is hydrogen, which tin can exist considered to be made upwardly of 2 seperate protons and electrons. There are two molecular orbitals for hydrogen, the lower energy orbital has its greater electron density between the ii nuclei. This is the bonding molecular orbital - and is of lower energy than the two 1s atomic orbitals of hydrogen atoms making this orbital more stable than two seperated diminutive hydrogen orbitals. The upper molecular orbital has a node in the electronic wave role and the electron density is depression between the ii positively charged nuclei. The energy of the upper orbital is greater than that of the 1s atomic orbital, and such an orbital is called an antibonding molecular orbital.
Normally, the two electrons in hydrogen occupy the bonding molecular orbital, with anti-parallel spins. If molecular hydrogen is irradiated by ultra-violet (UV) calorie-free, the molecule may absorb the free energy, and promote one electron into its antibonding orbital (s *), and the atoms will seperate. The energy levels in a hydrogen molecule can be represented in a diagram - showing how the ii 1s atomic orbitals combine to form ii molecular orbitals, ane bonding (s) and i antibonding (southward *). This is shown below - by clicking upon either the s or south * molecular orbital in the diagram - information technology will testify graphically in a window to the correct:
iii. Orbitals for selected molecules
This section illustrates pictorially molecular orbitals for several organic and inorganic molecules. If possible - the free energy level diagram is included and clicking upon the relelvant level will generate the accompanying molecular orbital in the correct-hand frame. Please choose from:- Saturated molecules
- Molecules with double bonds
- Molecules with triple bonds
- Molecules with electron lone pairs
- Conjugated and aromatic molecules
- Other interesting molecules
Saturated molecules
These are molecules in which all valence electrons are involved in the formation of unmarried bonds. There are no non-bonded lone pairs. These molecules are more often than not less reactive than either electron-rich or electron-deficient species, with all occupied orbitals having relatively low energies.
Methane:
The valence molecular orbitals of methane are delocalized over the entire nuclear skeleton - that is, information technology is non easy to assign whatever one orbital to a detail C-H bond. It is possible to see how complex the orbital construction becomes with the increment in energy. Methane has iv valence molecular orbitals (bonding), consisting of 1 orbital with one nodal plane (everyman occupied) and three degenerate (equal energy) orbitals that practise have a nodal plane.
For the energy diagram and pictorial view of the orbitals - please encounter beneath:

Ethane:
The ethane molecule has fourteen valence electrons occupying seven bonding molecular orbitals. Equally tin be seen from the energy diagram - four of the molecular orbitals occur as degenerate pairs. Similar in methane - the molecular orbitals of ethane prove increasing nodal structure with increasing orbital energy.
For the energy diagram and pictorial view of the orbitals - please come across below:

Molecules with double bonds
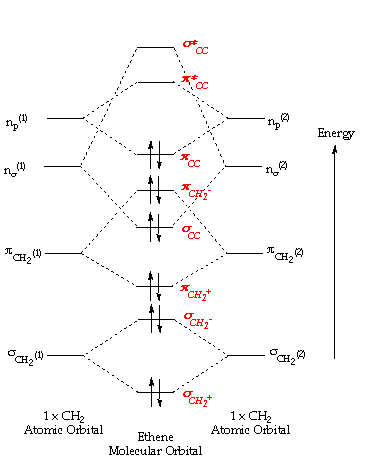
In molecules where the number of bonding electron pairs exceeds the number of unions between atoms, the actress electrons occupy college energy molecular orbitals than the orbitals establish in molecules where the number of bonding electron pairs equals the number of unions between atoms. These are double bonds, and the orbitals have a nodal plane containig the atoms sharing these p-blazon orbitals.Ethene:
The simplest alkene is ethene. Its chemistry is dominated by two "frontier orbitals", that is the Highest Occupied Molecular Orbital (Human) and the Lowest Unoccupied Molecular Orbital (LUMO). For the ethene orbital energy diagram these are shown as p CC for the HOMO, and p * CC for the LUMO.
An important belongings of the ethene molecule, and alkenes in full general is the existence of a loftier barrier to rotation about the C=C which tends to concur the molecule flat.
For the free energy diagram and pictorial view of the orbitals - please see below:
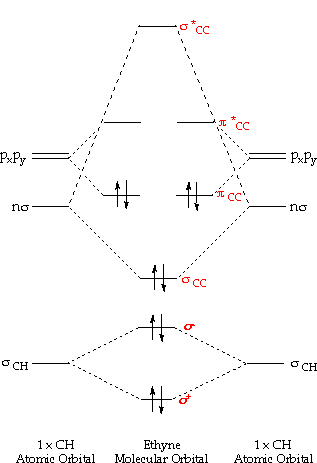
Molecules with triple bonds
Ethyne: For the energy diagram and pictorial view of the orbitals - please come across below:
Molecules with electron alone pairs
Hydrogen Fluoride: A elementary diatomic molecule is Hydrogen fluoride. In that location are eight valence electrons which occupy four molecular orbitals. The 2 highest energy MO'south are degenerate, are p-type and have no electron density associated with the hydrogen atom, ie. they are Non-Bonding Orbitals (NBO) and in Lewis Theory are represented as 2 "Alone Pairs". Another important difference betwixt Hydrogen Fluoride and previous molecules is that the electron density is not equally distributed nigh the molecule. There is a much greater electron density effectually the fluorine atom. This is because fluorine is an exremely electronegative element, and in each bonding molecular orbital, fluorine will take a greater share of the electron density.
For the energy diagram and pictorial view of the orbitals - delight see beneath:

Water:
In the h2o molecule the highest occupied orbital, ( 1b1 ) is non-bonding and highly localized on the oxygen atom, similar to the not-bonding orbitals of hydrogen fluoride. The adjacent everyman orbital ( 2a1 ) can be thought of as a non-bonding orbital, as it has a lobe pointing away from the two hydrogens. From the lower energy bonding orbitals, it is possible to run across that oxygen also takes more its "off-white share" of the total electron density.

Ammonia:
Ammonia has two pairs of degenerate orbitals, one bonding and one antibonding, and like hydrogen fluoride and water has a not-bonding orbital ( 2a1 ). This highest occupied orbital has a lobe pointing away from the three hydrogens, and corresponds to a lonely pair orbital localized upon the nitrogen, whereas the three lowest free energy MO'southward lead to the description of the three Northward-H bonds of the Lewis structure. The lone pair is relatively high in energy, and is responsible for the well known Lewis base properties of ammonia.

The side by side molecule in the series HF, H2O and HthreeNorth, is H4C (methane) - which was discussed earlier - and different the other three molecules has no not-bonding orbitals.
Conjugated and effluvious molecules
p bonds in close proximity will ofttimes collaborate. Some of the delocalized molecular orbitals that result will exist stabilized, while others will be destabilized. The individual combinations may be polarized, providing an increment in wave function amplitude on some centers at the expense of a decrease in amplitude on others. This gives rise to the possibility of more varied reactivity patterns than are observed for simple alkenes. Aromatic molecules exhibit a wide range of reactivity patterns toward both electron rich and electron deficient species. These mainly depend on the structures and energies of the borderland p-type molecular orbitals, the Human being and LUMO. Except for non-bonded lone pairs, the s framework plays little office in the overall reactivity.
trans-1,3-Butadiene:
The energies of the p-molecular orbitals of conjugated molecules like butadiene, (see below) - occur in pairs, with their energies equal to (a±10 b), where a and b are constants. For each bonding orbital of and free energy a-x b there is a corresponding antibonding orbital of energy a+10 b. The p-molecular orbitals are extended over the whole molecule.
For butadiene, the p manifold contains iv electrons, leading to an electronic configuration of p 1 2 p 2 2.
For the free energy diagram and pictorial view of the p-molecular orbitals - please come across beneath:
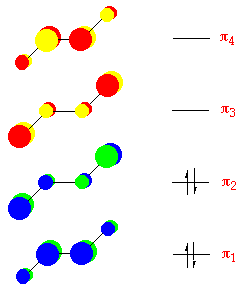
Allyl radical
The radicals allyl:
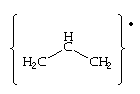
and pentadienyl:
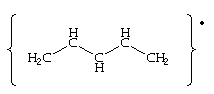
have the aforementioned arrangement of p-orbitals, (ie. the occur in pairs of free energy a±x b), but because there is an odd number of carbon atoms in the conjugate concatenation, at that place must be a non-bonding orbital with free energy ten=0. As well, considering of the pairing backdrop of the p-molecular orbitals of conjugated chains, at that place will be a node at every alternate carbon atom in the non-bonding orbital. This is of import for the unpaired electron of allyl, which will occupy this not-bonding orbital. If an electron is added to the allyl radical to course the anion, the negative charge will appear at the terminal carbon atoms. If the unpaired electron is removed forming the cation, the resulting positive accuse is also spread over the termial carbon atoms.
There are three p-molecular orbitals for allyl, the p 1 is bonding, the p 2 orbital is non-bonding and the p iii is anti-bonding. In the neutral allyl species - there are a total of seventeen valence electrons - of which three fill the p-orbital manifold. A pictorial representation of the energy diagram for the neutral, cationic and anionic allyl species are shown below - (orbitals are shown only for the cationic species):
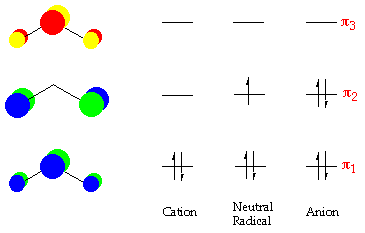
In the pentadienyl anion, the negative charge is centred on the carbon atoms in the 1,3 and 5 position - similarly with the positive charge for the cation.
These ions are represented in resonance theory as two or three canonical forms:
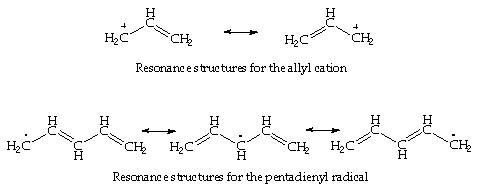
The delocalisation of p-electrons is associated with a lowering of the orbital free energy. Therefore the total energy of the occupied p-orbitals of butadiene is lower in energy then two isolated ethene-blazon double bonds. Farther delocalisation of p-electrons occurs in aromatic hydrocarbons. A effigy showing the comparative energy levels of the p-orbitals of the cyclic molecules CnHn , for n=3-6, is shown below:
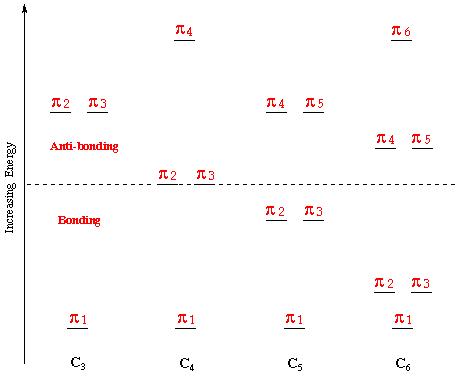
In all cyclic polyenes (CnorthwardHn ), the p-molecular orbitals occur in degenerate pairs, except for the everyman p-orbital, and for the circadian polyenes with even numbers of carbon atoms, the highest p-orbital (see to a higher place).
Cyclobutadiene:
From the cyclic polyene diagram - the square molecule cyclobutadiene (C4Hiv ) has four p-orbitals, a bonding orbital ( p ane ), two degenerate non-bonding orbitals ( p 2 and p three ) and an anti-bonding orbital ( p 4 ). Iv electrons are placed into these four orbitals; twon into the bonding orbital, and i each with parallel spins into the degenerate non-bonding orbitals (Hund's dominion) - encounter below:
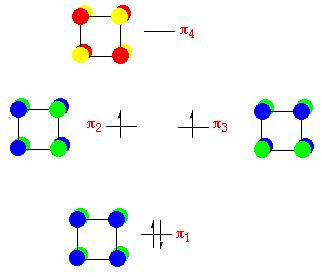
There is no reason to expect cyclobutadiene to be foursquare, theoretically calulations testify an oblong with two double bonds structre has lower energy. Experimentally information technology is shown that cyclobutadiene acts a very strained cyclo-olefin, rather than as a bi-radical species.
Cyclopentadiene:
This molecule is a relatively acidic hydrocarbon, and the anion is formed past the treatment of cyclopentadiene with a strong base. From the cyclic polyene diagram it tin be seen that cyclopentadiene has iii p bonding orbitals which are delocalised over the five carbon atoms. The uppermost p bonding orbitals are a degenerate pair, and are the highest occupied molecular orbitals (HOMO's).
These orbitals are much higher in energy than those in neutral aromatic species such every bit benzene, indicating that this anion is far more susceptible t attack by electrophiles. The anion is far more capable of coordinating to transition metals with available empty d-orbitals. The anion has six p-electrons, making the system effluvious. The 6 electrons are arranged equally in the diagram below:
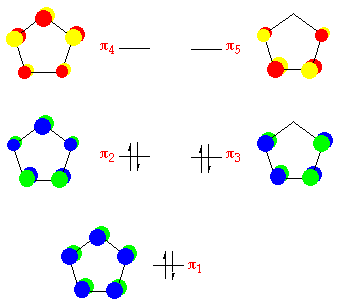
Benzene:
Benzene is the archetypal aromatic chemical compound. It has a symmetrical p system and so is not over reactive on any i site. From the cyclic polyene diagram it tin can exist seen that benzene has half dozen p-molecular orbitals, (which incorporate the vi p-electrons), 3 bonding and three anti-bonding. The upper bonding degenerate pair of orbitals are the HOMO's of benzene. The p orbital manifold, is shown below - but as well of interst is that the design of the p orbitals is repeated inside the southward system. The s functions - similar the p orbitals are delocalized throughout the carbon skeleton.
The half-dozen p electrons are arranged every bit in the diagram below:
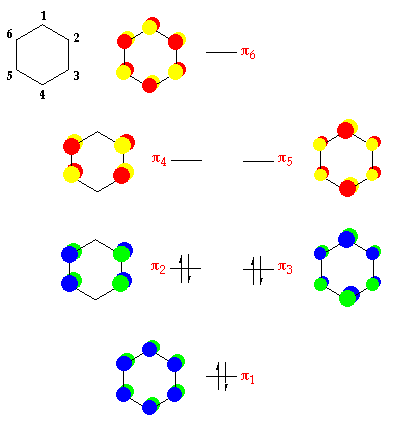
Bonding in benzene
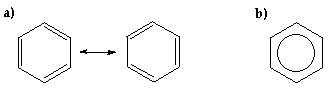
From the above diagram it can be seen that the everyman lying orbital, p i , the orbital coefficients are such that the bonding charachter betwixt each pair of adjacent carbon atoms is equal. In p 2 bonding only occurs between atoms C2 and Cthree and between Cfive and C6 since the coefficients on C1 and C4 are nil. In p 3 , C1, which is bonded to C2 and Csix and Cfour is bonded to Cthree and C5, there are anti-bonding interactions between Cii and C3 and betwixt C5 and Cvi. Therefore if we consider the pair of orbitals p 2 and p 3 the contribution to the C-C p bonding is equal for each bond. Since in that location are three occupied bonding orbitals and six CC linkages - the p bond social club is i/2. This clarification is in accord with the two resonating mesomeric forms (or Kekulé structures in a) beneath in which single and double bond characters alternate around the band. Conventionally, the diagram in b) is used to show that the six electrons are delocalized effectually the ring:
Other interesting molecules
Aii MoleculesNitrogen:
This molecule has ten electrons. The atomic orbitals combine to produce the following molecular orbital diagram:
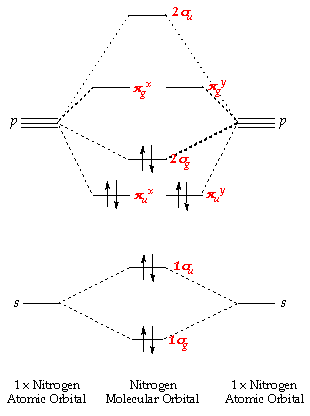
Here the 2p g orbital is occupied by two electrons to give a total bail order of three. This corresponds well with the Lewis construction (  ), although the orbital arroyo tells us that there is one s and two p.
), although the orbital arroyo tells us that there is one s and two p.
Oxygen:
This molecule has twelve electrons, two more than nitrogen - and these extra two are placed in a pair of degenerate p g orbitals. The atomic orbitals combine to produce the following molecular orbital diagram:
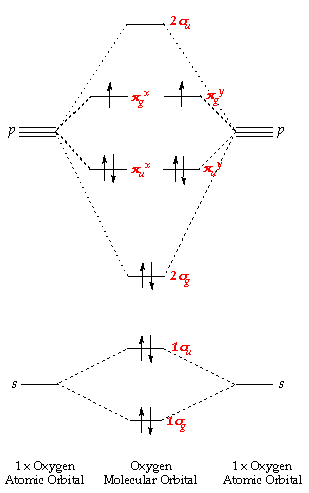
Comparing of the higher up energy level diagram wit hthat for nitrogen - you tin can see that the 2southward g level lies lower than p u. Here, nosotros are starting to fill the anti-bonding orbitals originating from the p orbital interactions and so the bond order decreases from 3 to two.
The lowest energy arrangment (Hund's rule) - has a single electron, each with parallel spins, in each of the p m x and p thou y orbitals. This produces a paramagnetic molecule, with a double bond and has ii unpaired electrons.
© W. Locke and the ICSTM Department of Chemical science 1996-97.
Source: https://www.ch.ic.ac.uk/vchemlib/course/mo_theory/main.html
Posted by: martinezdiente.blogspot.com


0 Response to "How To Draw Atomic And Molcular Orbitals"
Post a Comment